Join us at 25th World Congress on Pharmaceutical Biotechnology scheduled on November 18-19, 2021 Barcelona, Spain.
Theme: Emerging technologies that shaping the future of the pharma industry
The conference series is passionately welcoming each one of you to participate to meet your peers and subject matter experts in the global physical conference
25th World Congress on Pharmaceutical Biotechnology has been scheduled during November 18-19, 2021 at Barcelona, Spain.
The conference deliberations will be on the theme Emerging technologies that shaping the future of the pharma industry
Pharma Biotech 2021 is going to be a remarkable event that aims to bring together an international mixture of various pharma researchers, scholars, pharmacists, scientists, pharma companies or industries, directors, leading universities, students, faculty, related associations, and research institutions making the conference an ideal platform for sharing, exchanging and exploring latest findings, clinical experiences, research results, novel strategies, and advancement of emerging technology applications for pharmaceutical innovation and modernization across the globe.
In addition to keynote presentations and a variety of poster presentations along with workshops and interactive sessions and lectures by world-renowned invited speakers. We will be offering special sessions, and hands-on workshops would be interested in the audience.
Join us and utilize the immense opportunity to promote communication and collaboration with your peers from academia and industry: We believe that you will benefit from the conference that brings a new era for innovations in the field of pharmaceutical science and the interaction with colleagues worldwide will stimulate a creative exchange of ideas.
Abstract submission
“Pharama Biotech 2021” would like to call experts, industrial excellences, academic researchers and students to submit a brief note of their work as an abstract which must comprise of 150 to 350 words. Authors should choose the given scientific sessions and the subject matters while submitting the abstracts in the portal, new sessions are also welcome to be recommended by the speakers.
(Please note that, only accepted abstracts will be published in the Pharma biotech 2021 proceedings)
Why to become a member?
There are so many great ways to learn and sharpen your skills in your specific field. One of the best ways to meet and communicate with many subject matter experts from different regions of the world which is always a common demand for every participant to build up a phenomenal collaboration, growth and market analysis of the concerned research.
Benefits of attending the conference:
-
All the accepted abstracts will be published in the respective Conference Series LLC LTD International journals
-
Global Recognition of Scholars
-
Facilitate sharing and collaboration between world class researchers
-
Establishing academic and professional relationships
-
Improves confidence in presenting research on an international platform.
-
Interacting with expertise in their respective departments
-
To Learn New Tips & Tactics in the field
-
For Global Networking and Brand Establishment
-
Exposure to the Upcoming Business Trends
-
Assessing the Research and Communication of Research Discoveries
Targeted audience:
-
All the Stake Holders (both Academia & Industry) of the Sector
-
Researchers & Innovators, Scientists
-
Pharmaceutical & Biotechnology Companies
-
R & D Labs
-
Doctors and scholars
-
Pharmacists and Medical representatives
-
Professors and Students
-
Universities and Research Institution
-
Noble laureates in Health Care and Medicine
-
Supply Chain and Manufacturing Companies
-
Related Associations, Societies, & Professional Bodies
-
Experts Looking for Collaborative Work
-
Product Developers
-
Solution Providers
-
Bio instruments Professionals
-
Bio-informatics Professionals
-
Industry directors and Leaders
-
Sales & Marketing Professional
The research process to develop COVID-19 drug that would reduce the severity of coronavirus disease (COVID-19). Coordinating with academic and industry researchers to speed development of vaccines, antiviral drugs, and post-infection therapies.
-
Covid-19 impact on worldwide pharma sector
-
Drug evaluation and research
-
Medical treatment options for covid-19
-
Nature medicine
-
Antiviral medications
-
Recovery trial
-
Novel antibody drugs
-
Convalescent plasma
Vaccines contain the same germs that cause particular infectious diseases. But they have been either killed or weakened to the point that they don’t make you sick. The vaccine stimulates your immune system to produce antibodies.
-
Types of Vaccines
-
Novel Vaccine Drug Delivery Systems
-
Vaccine Safety and efficiency,
-
Vaccine Research & Development
-
Anti-Microbial Resistance
-
Vaccines Bioprocessing And Manufacturing
-
Current Research & Future Challenges in Vaccines
-
Vaccines against Drugs
Technology has been a significant operator regarding advancements toward drug discovery. Automation, nanofluidics, imaging, software, and assay technologies have performed a principal role in obtaining better data, faster
-
Innovative trials
-
Novel Drug Delivery Systems
-
Cancer Targeted Drug Delivery
-
Advances in Imaging
-
Body Sensors
-
AR and VR
-
3d Printed drugs
Research on data or samples of tissue from people, aimed at evaluating a medical, surgical, or behavioral intervention. Researchers find out if a new treatment, like a new drug or diet or medical device through Clinical Trials and Clinical Research.
-
Novel Developments
-
Drug Targeting and Design
-
Current Therapeutic Drugs Treating COVID-19
-
Clinical Trial Protocols
-
Prevention trials, Screening trials, treatment trials.
-
Clinical Research and Statistics
-
Safety, Efficacy and Toxicity of Drug
-
Diversity in Industrial Clinical Trials
Bioimaging is a field of biomedicine and bioengineering that includes the advancement and utilization of imaging technologies and computational software tools in the production of pharmaceutical specimens.
Biomarkers is a biological molecule detected in blood or other body fluids, or tissues is a sign of a common or unusual process, or a condition or disease. A biomarker may be used to examine wherewith the body reacts to medication for a disease or condition.
A biosimilar drug has a structure that is similar but not exactly the same, as a brand name biologic drug that already approved.
-
Magnetic resonance imaging (MRI),
-
Subclinical manifestation,
-
DNA sequence
-
Functional imaging
-
Ultrasound imaging
-
Clinical trails
-
Computed tomography
-
Positron emission tomography (PET),
-
Bio imaging techniques
Artificial intelligence (AI) is the simulation of human intelligence methods through machines, especially computer systems. Pharma industries implementing artificial intelligence for higher productivity, improved efficiency, and faster production of life-saving drugs.
-
Quality control
-
Waste reduction
-
Design optimization
-
Process automation
-
Pharma packaging
Pharmaceutical Formulation in pharmaceutics is the process in which different chemical substances including the energetic drug are combined to manufacture a final tablet or a pill.
Industrial bioprocessing mainly deals with the design, production, fabrication of drugs in the biological treatment of wastewater. Industrial bioprocessing are also involved in the designing of spectrums in bioreactors.
-
Value-added material
-
Preparation, production, and purification.
-
Upstream process
-
Bioprocess technology
-
Therapeutic substances
-
Encompasses bioreactor vessels
-
Therapeutic substances
-
Encompasses bioreactor vessels
Nanomedicine has attracted scientists because of its enormous properties such as its petite size, capacity to pack various drugs and large surface area, and strength to increase the absorption of conjugated.
Biotechnology products for therapeutic use incorporate a very distinctive array of products, including growth factors, cytokines, hormones, receptors, clotting factors, monoclonal antibodies, vaccines, gene transfer products, and tissue/organ grafts.
-
Conventional therapies
-
Cell therapies
-
DNA vaccines
-
Experimental Therapeutics
-
Enzymes
-
Biomaterials-based approaches
-
Novel bioactive compounds
-
Medicinal plants
Cosmetics have an impact of Biotechnology in many ways. Cosmetics manufacturing companies use biotechnology both to discover and manufacture components of cosmetics and to evaluate the effects of natural and synthetic compounds on skin and skin changes associated with aging.
-
Botox.
-
Use of biotechnology-derived ingredients
-
Genetic profiling
-
Skin care
-
Biofermentation
Drug regulation is the control of drug usage by international agreements and/or by regulatory authorities. Quality assurance of pharmaceuticals and Quality Management that ensures products manufactured upon the quality standards and appropriate or not to their proposed use
-
Principles for pharmaceutical products
-
Heating ventilation and air-conditioning systems for non-sterile pharmaceutical dosage
-
Good manufacturing practices
-
Inspection
-
Hazard and risk analysis
-
Sampling
Pharmacogenomics is the study of genes and their functions that affect a person's response to drugs. Several technologies, new diagnostic procedures, and therapeutic products to develop genetic variances for safe medications and doses that will be tailored to a person's genetic makeup. Personalized medicine is a customized medical treatment to the individual features of each patient.
Biotechnology has assisted researchers to assume cancer in many ways such as Gene profiling, Genome analysis, cell culture, culturing transgenic cell lines, and especially identification of new biomarkers for detection of risk and progression of cancer
-
New approaches
-
Genetic profile
-
Targeted Therapies
-
Cancer Vaccines
-
quality assured diagnostics
-
Cancer Drug Discovery
-
Cancer Diagnostics advances
-
Imaging in cancer
-
Nano-medicine in cancer diagnostics and treatment
Pharmaceutical engineering focuses on discovering, designing, formulating, and improving manufacturing facilities that produce drugs. It uses some areas of biomedical engineering, chemical engineering and pharmaceutical sciences.
Biopharmaceutical downstream processing (DSP) refers to the recovery and purification of a drug substance (DS) from natural sources, such as animal or bacterial cells, biological materials, such as cells, tissue culture fluid, or plant tissues to derive pure and homogeneous protein product.
-
Chromatographic purification in downstream processing
-
Gel filtration (GF) chromatography
-
Bio-separation
-
SDS-PAGE
In growing pharma industry, the necessity of robotics and automated systems including sensors, an intelligent algorithm, and an actuator in clinical trials and drug discovery, and even in the growth of laboratory.
-
Robots for vaccine production
-
Cold Chain robotic handling
-
Freezer robot handling
-
Automated testing sensors
-
Automated pharmaceutical test kit
Drug laboratories are sites where the new drug and detecting techniques were developed and tried. With the growing world, several technologies were developed to test and diagnose and implement the drugs.
Pharmacoinformatics interface of technology with the practice of pharmacy where the new drug development and modernization of drug discoveries using various bioinformatics technologies and statistical methods and algorithms like artificial Neural Networks
Combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues is known as Tissue engineering. DNA technology is the combination of DNA molecules from two different species. These recombined DNA molecules are inserted into an organism to generate new genetic combinations
-
3D functional tissue
-
Tissue regeneration
-
Developments
-
Latest technologies
-
Three-dimensional (3D) scaffolds
-
Cloning strategies, E. Coli transformation
With the growing world, the pharma industry developing day by day in all aspects. the total global pharmaceutical market is valued at about 1.27 trillion U.S. dollars, as of end-2020. This is a notable uptick from 2001 when the market was evaluated at just 390 billion U.S. dollars.
With new companies, new products, and new research centers, spain is a world-class contender in the biotech industry.
Spain represents more than 2.1% of the world's total GDP and has experienced a striking 14-year streak of economic growth beyond the mark of 3%
Spain ranks 4th in the EU in terms of the number of laboratories, with 183 pharmaceutical laboratories.
Pharmaceutical manufacturing research market report: 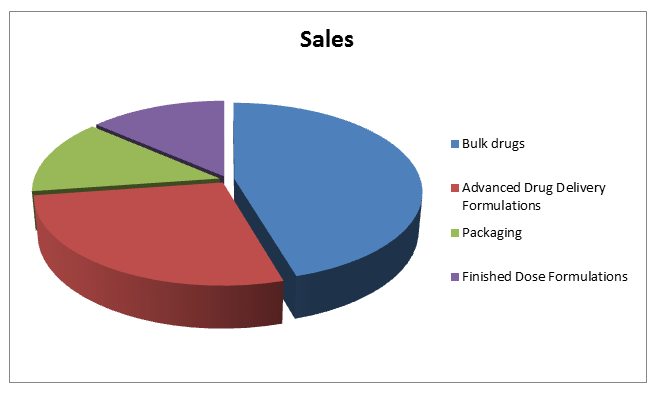
Technologies that have a greatest impact on pharma industry 2021:
Pharmaceutical engineering focuses on discovering, designing, formulating, and improving manufacturing facilities that produce drugs, 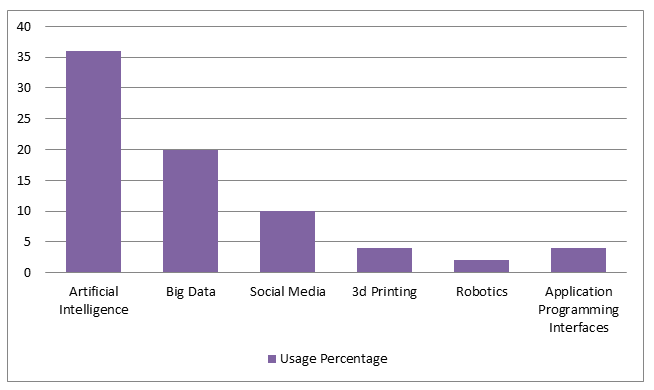
Covid 19 impacts on the growth of drug market:
Researchers and physicians in several countries are focusing on various other existing drugs to examine their potential to treat COVID-19.
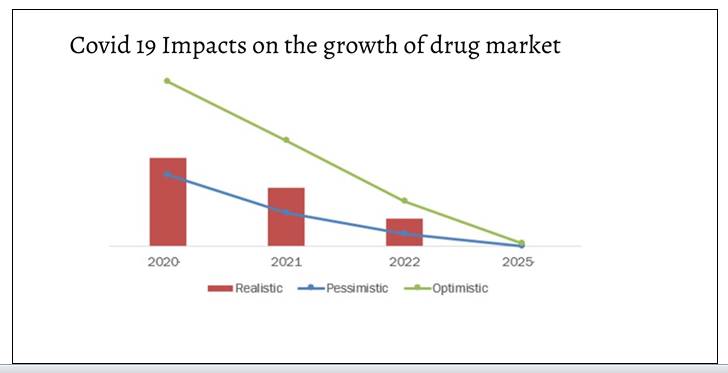
Pharmaceutical industry in the coming years by delivering productivity improvements and efficiencies across the entire pharma value chain
Even though there are still some barriers for pharmaceutical companies in Spain, such as the Spanish Government drugs’ prices regulation within the public health system, spain continues to be a very attractive market for pharmaceutical companies worldwide and we encourage canadian clients to explore this interesting market.













